Introduction
The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); that is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample of the liquid (or solid) in a closed container. The vapor pressure of a liquid varies with its temperature. As the temperature of a liquid or solid increases its vapor pressure also increases.
Vapour pressure and intermolecular force:
Some of the factors that affect vapor pressure are;
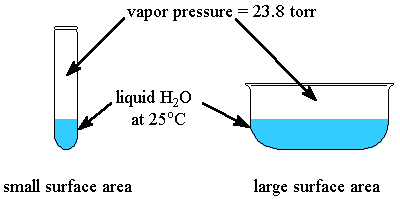
- Surface Area: the surface area of the solid or liquid in contact with the gas has no effect on the vapor pressure.
- Types of Molecules: the types of molecules that make up a solid or liquid determine its vapor pressure. If the intermolecular forces between molecules are:
- relatively strong, the vapor pressure will be relatively low.
- relatively weak, the vapor pressure will be relatively high.
Knowing this useful background information, we will investigate intermolecular forces further. We carried out an experiment where different compounds (each group investigated one compound) were put under different temperatures and the vapor pressure was measured. The results will be highly affected by the intermolecular forces of attraction the particles have. Let's see how this happens, but before let's state our objectives during this session.
Objective 1. To improve practical skills – use of Schlenk tube, pressure sensors, vacuum line.
Objective 2. To investigate the structure and properties of one particular chemical.
Objective 3. To investigate the effect of temperature on vapour pressure.
Objective 4. To compare results with other groups (with other chemicals) and relate them to “intermolecular forces”.
Objective 2. This video is an explanation on the molecule of buthanol:
Objective 3. Here is the graph that shows the relationship between pressure and time. Keep in mind that as time goes by, the temperature increases, the last bit of this function should be the second last, so we could see the relationship a bit clearer; this is because the temperature there would have been 25 °C, while the other two bits show what happens to the pressure when the temperature is changed to 15 °C and 35 °C. If we had plotted a graph showing pressure vs. temperature, then the order of the results would not have mattered.
Graph of the data we obtained:
In this graph it is shown how the pressure changed with time but the reason why it changed is because of the change in temperature.When the temperature of the substance, buthanol, increased, the pressure did too because the particles got more energy to move around the container. As the particles had more energy, they collided more strongly and quickly against each other and against the walls of the container which lead to a higher pressure. Gay Lussac's law explains this relation between temperature and pressure, as this law says that temperature and pressure directly proportional. This means that as the temperature increases, the pressure does too.
Also, as buthanol was heated up, the particles had more energy to turn into a gas and more vapour pressure was created.
Improvements in the method:
As we can notice in the graph, at a certain point, we skipped one of the temperatures. When we realised that we skipped that temperature (25ºC), we went back and did it after heating buthanol 35ºC, so that is why on the right of the graph the pressure suddenly increases and at the end decreases because as we mentioned earlier, temperature and pressure are directly proportional. We should have followed the order of the temperatures to not have drastic increases of temperature, so this could be improved.
As we can notice in the graph, at a certain point, we skipped one of the temperatures. When we realised that we skipped that temperature (25ºC), we went back and did it after heating buthanol 35ºC, so that is why on the right of the graph the pressure suddenly increases and at the end decreases because as we mentioned earlier, temperature and pressure are directly proportional. We should have followed the order of the temperatures to not have drastic increases of temperature, so this could be improved.
Before going through the results, let's recap the types of intermolecular forces we have studied.
Van der Waals. It happens because electrons move randomly, and at some point, there can be more electrons on one side of the molecule. Electrons from other molecules nearby will be repelled from this negative charge, and since opposite charges attract . It is the weakest of all intermolecular forces
Permanent dipole-dipole. Occurs between polar molecules, so it is stronger than Van der Waals.
Hydrogen bonding. The theory behind it would be the same as a permanent dipole-dipole interaction but it is a bond that occurs between hydrogen and a FON particle; that is fluorine, oxygen and nitrogen. It is the strongest of all types of bonding.
Now, let's go one by one through the results we obtained.
In the case of pentane, it is the only molecule that has Van der Waals forces of attraction as it's one and only intermolecular force. We must recap that Van der Waals (also known as London dispersion forces) is the weakest of all forces of attraction. So, even if pentane is a very big and long molecule, when compared to the rest of molecules, it has the lowest boiling point, as well as the highest vapor pressures.
Moving onto ethyl acetate, this molecule seems to have two types of intermolecular forces; Van der Waals and permanent dipole-dipole. The molecule is quite big and it has permanent dipole-dipole forces of attraction because oxygen is quite electronegative. However even if oxygen is one of the FON elements, since it is not bonded to hydrogen but carbon, then we can only call this a dipole-dipole interaction. As a result, the boiling point becomes higher when compared to pentane's and the vapor pressures become lower as well.
If we examine butyl acetate, we will first notice that it has the highest boiling point of them all. This is because it has two types of forces present in a very strong way. It has strong Van der Waals forces because it is a big and large molecule. Moreover, since oxygen is very electronegative, this makes the Van der Waals forces of attraction stronger as electrons will concentrate in that area. Furthermore, it also has strong permanent dipole-dipole because oxygen is very electronegative.
Moreover, the case of 1-Butanol is an interesting one. It has a relatively high boiling point, yet the vapor pressure is extremely low. This could be due to the arrangement of particles. The permanent dipole-dipole interaction in butyl acetate outweighs the occurring hydrogen bonding in 1-Butanol, therefore causing a higher boiling point in butyl acetate.
Last but not least, propyl acetate. It's got Van der Waals forces of attraction and permanent dipole-dipole interaction. The vapor pressures are relatively low but not lower than butyl acetate's. This means that the attraction is weaker, also causing a lower boiling point than butyl acetate. The permanent dipole-dipole would be quite as strong one as oxygen is very electronegative.


Mr. Canning, this is what you commented on another blog post, which we deleted to avoid confusion.
ResponderEliminar"This is missing a large portion of what is required!
Formative:
B - 2 The writing here is correct but where is the intro, objectives etc.... (all is explained on the wikispace. More detail is required in the comparison of the chemicals, their boiling points and intermolecular forces.
D - 3 You carried out the investigation well but have not discussed the objectives or procedure.
E - 4 The table is good but where is the Loggerpro graph of your vapour pressures at different temperatures for your group´s chemical?"
We have now put everything together.
Summative:
ResponderEliminarB - 6 This is an excellent effort. The level of detail is outstanding and well communicated. You should always include references but I will let you off as there has clearly been a lot of effort made in this blog.
E - 6 The graph and table are clear and well explained.
Excellent effort!